Plastics: The Basics
Plastics are polymers. What is a polymer? The most simple definition of a polymer is something made of many units. Think of a polymer as a chain. Each link of the chain is the "-mer" or basic unit that is usually made of carbon, hydrogen, oxygen, and/or silicon. To make the chain, many links or "-mers" are hooked or polymerized together. Polymerization can be demonstrated by linking countless strips of construction paper together to make paper garlands or hooking together hundreds of paper clips to form chains, or by a string of beads. Polymers have been with us since the beginning of time. Natural polymers include such things as tar and shellac, tortoise shell and horns, as well as tree saps that produce amber and latex. These polymers were processed with heat and pressure into useful articles like hair ornaments and jewelry. Natural polymers began to be chemically modified during the 1800s to produce many materials. The most famous of these were vulcanized rubber, gun cotton and celluloid. The first truly synthetic polymer produced was Bakelite in 1909 and was soon followed by the first synthetic fiber, rayon, which was developed in 1911.
The Structure of Polymers
Many common classes of polymers are composed of hydrocarbons. These polymers are specifically made of small units bonded into long chains. Carbon makes up the backbone of the molecule and hydrogen atoms are bonded along the backbone. Below is a diagram of polyethylene, the simplest polymer structure.
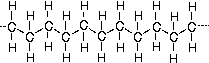
There are polymers that contain only carbon and hydrogen. Polypropylene, polybutylene, polystyrene, and polymethylpentene are examples of these.
Even though the basic makeup of many polymers is carbon and hydrogen, other elements can also be involved. Oxygen, chorine, fluorine, nitrogen, silicon, phosphorous, and sulfur are other elements that are found in the molecular makeup of polymers. Polyvinyl chloride (PVC) contains chlorine. Nylon contains nitrogen. Teflon contains fluorine. Polyester and polycarbonates contain oxygen. There are also some polymers that, instead of having a carbon backbone, have a silicon or phosphorous backbone. These are considered inorganic polymers. One of the most famous silicon-based polymers is Silly Putty.
Molecular Arrangement of Polymers
Think of how spaghetti noodles look on a plate. This is similar to how polymers can be arranged if they lack a specific for or are amorphous. Controlling and quenching the polymerization process can result in amorphous organization. An amorphous arrangement of molecules has no long-range order or form in which the polymer chains arrange themselves. Amorphous polymers are generally transparent. This is an important characteristic for many applications such as food wrap, plastic windows, headlights, and contact lenses.
Obviously not all polymers are transparent. The polymer chains in objects that are translucent and opaque are in a crystalline arrangement. By definition a crystalline arrangement has atoms, ions, or in this case, molecules in a distinct pattern. You generally think of crystalline structures in salt and gemstones, but not in plastics. Just as quenching can produce amorphous arrangements, processing can control the degree of crystallinity. The higher the degree of crystallinity, the less light can pass through the polymer. Therefore, the degree of translucence or opaqueness of the polymer is directly affected by its crystallinity.
Scientists and engineers are always producing better materials by manipulating the molecular structure that affects the final polymer produced. Manufacturers and processors introduce various fillers, reinforcements, and additives into the base polymers, expanding product possibilities.
Characteristics of Polymers
Polymers are divided into two distinct groups: thermoplastics and thermosets. The majority of polymers are thermoplastic, meaning that once the polymer is formed it can be heated and reformed over and over again. This property allows for easy processing and facilitates recycling. The other group, the thermosets, can not be remelted. Once these polymers are formed, reheating will cause the material to scorch.
Every polymer has very distinct characteristics, but most polymers have the following general attributes.
Polymers can be very resistant to chemicals. Consider all the cleaning fluids in your home that are packaged in plastic. Reading the warning labels that describe what happens when the chemical comes in contact with skin or eyes or is ingested will emphasize the chemical resistance of these materials.
Polymers can be both thermal and electrical insulators. A walk through your house will reinforce this concept, as you consider all the appliances, cords, electrical outlets and wiring that are made or covered with polymeric materials. Thermal resistance is evident in the kitchen with pot and pan handles made of polymers, the coffee pot handles, the foam core of refrigerators and freezers, insulated cups, coolers and microwave cookware. The thermal underwear that many skiers wear is made of polypropylene and the fiberfill in winter jackets is acrylic.
Generally, polymers are very light in weight with varying degrees of strength. Consider the range of applications, from toys to the frame structure of space stations, or from delicate nylon fiber in pantyhose or Kevlar, which is used in bulletproof vests.
Polymers can be processed in various ways to produce thin fibers or very intricate parts. Plastics can be molded into bottles or the bodies of a cars or be mixed with solvents to become an adhesive or a paint. Elastomers and some plastics stretch and are very flexible. Other polymers can be foamed like polystyrene (StyrofoamTM) and urethane, to name just two examples. Polymers are materials with a seemingly limitless range of characteristics and colors. Polymers have many inherent properties that can be further enhanced by a wide range of additives to broaden their uses and applications.
In addressing all the superior attributes of polymers, it is equally important to discuss some of the difficulties associated with the material. Plastics deteriorate but never decompose completely, but neither does glass, paper, or aluminum. Plastics make up 9.5 percent of our trash by weight compared to paper, which constitutes 38.9 percent. Glass and metals make up 13.9 percent by weight.
Applications for recycled plastics are growing every day. Recycled plastics can be blended with virgin plastic (plastic that has not been processed before) to reduce cost without sacrificing properties.
Recycled plastics are used to make polymeric timbers for use in picnic tables, fences, and outdoor toys, thus saving natural lumber. Plastic from 2-liter bottles is even being spun into fiber for the production of carpet.
An option for plastics that are not recycled, especially those that are soiled, such as used microwave food wrap or diapers, can be a waste-to-energy system (WTE).
The controlled combustion of polymers produces heat energy. The heat energy produced by the burning plastics not only can be converted to electrical energy but helps burn the wet trash that is present. Paper also produces heat when burned, but not as much as plastics. On the other hand, glass, aluminum and other metals do not release any energy when burned.
To better understand the incineration process, consider the smoke coming off a burning object and then ignite the smoke with a Bunsen burner. Observe that the smoke disappears. This is not an illusion, but illustrates that the by-products of incomplete burning are still flammable. Incineration burns the material and then the by-products of the initial burning.
Polymers affect every day of our life. These materials have so many varied characteristics and applications that their usefulness can only be measured by our imagination. Polymers are the materials of past, present, and future generations.
Resin Identification Code
The Society of the Plastics Industry, Inc. (SPI) introduced its voluntary resin identification coding system in 1988 at the urging of recyclers around the country. A growing number of communities were implementing recycling programs in an effort to decrease the volume of waste subject to rising tipping fees at landfills. In some cases, test programs were driven by state-level recycling mandates. The SPI code was developed to meet recyclers' needs while providing manufacturers a consistent, uniform system that could apply nationwide. Because municipal recycling programs traditionally have targeted packaging - primarily containers - the SPI coding system offered a means of identifying the resin content of bottles and containers commonly found in the residential waste stream. Recycling firms have varying standards for the plastics they accept. Some firms may require that the plastics be sorted by type and separated from other recyclables; some may specify that mixed plastics are acceptable if they are separated from other recyclables; while others may accept all material mixed together. Not all types of plastics are generally recycled, and recycling facilities may not be available in some areas.


